The difference between chlorine and chlorine dioxide is that the oxidation state of chlorine atom in chlorine gas is zero whereas the oxidation state of chlorine atom in chlorine dioxide is 4.
Chlorine dioxide at room temperature.
It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat and after severe exposure edema filling with fluid.
It does not occur naturally in the environment.
Clo 2 another strong oxidizing agent.
It is a strong oxidizing agent that under oxidant demand conditions is likely to reduce to chlorite casrn 7758 19 2.
Chlorine perchlorate can also be made by the following.
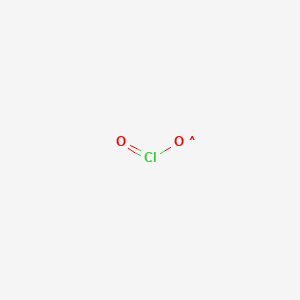
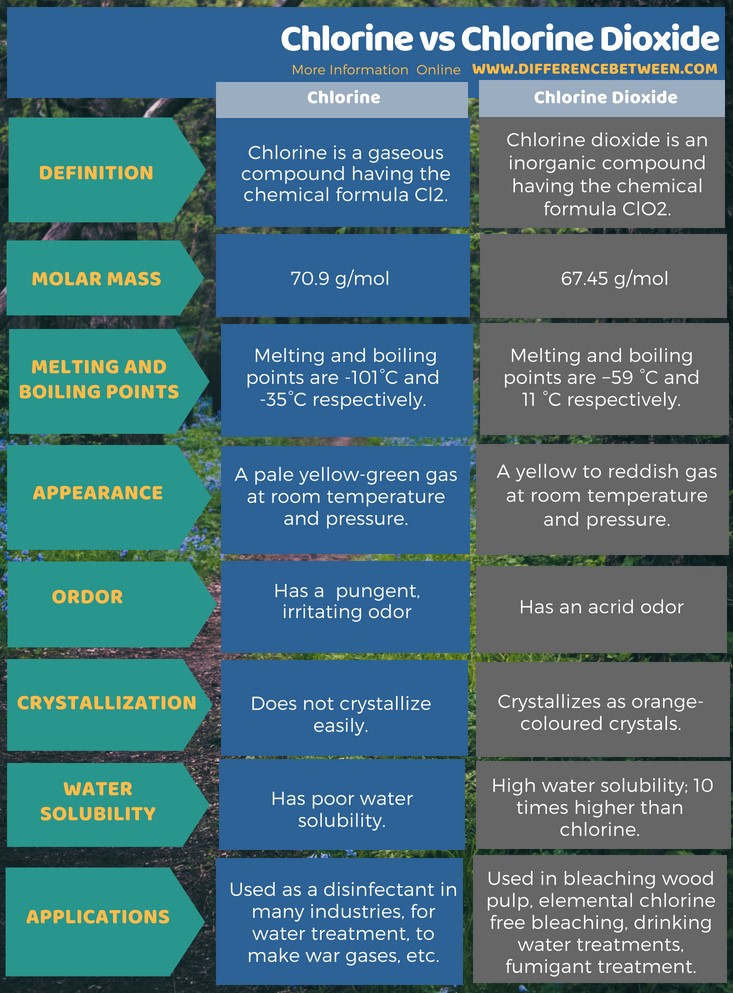
Chlorine dioxide is a chemical compound with the formula clo 2 that exists as yellowish green gas above 11 c a reddish brown liquid between 59 c and 11 c and as bright orange crystals when colder.
Chlorine and chlorine dioxide are gaseous compounds at room temperature and pressure.
Chlorine perchlorate is a chemical compound with the formula cl 2 o 4 this chlorine oxide is an asymmetric oxide with one chlorine atom in 1 oxidation state and the other 7 with proper formula cloclo 3 it is produced by the photolysis of chlorine dioxide clo 2 at room temperature by 436 nm ultraviolet light.
It becomes a liquid at 34 c 29 f.
It is a reddish to yellowish green gas at room temperature that dissolves in water.
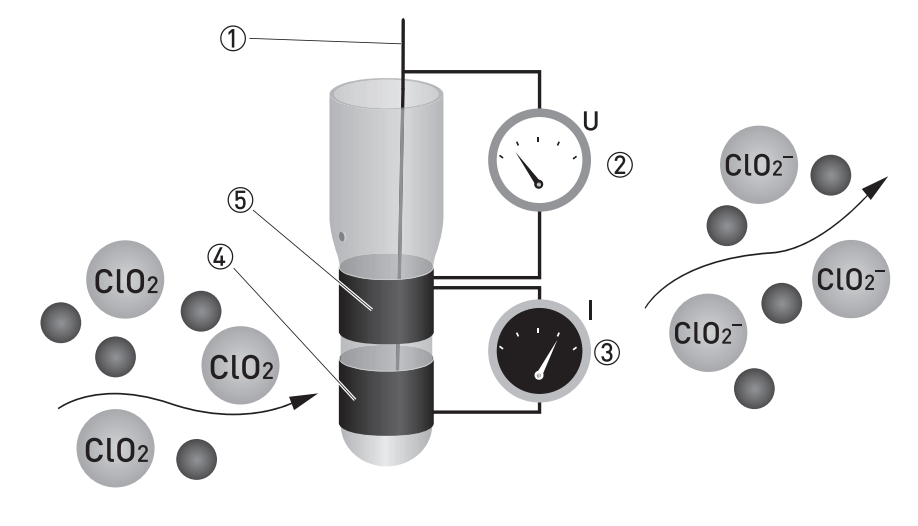
It is an oxidizing agent able to transfer oxygen to a variety of substrates while gaining one or more electrons via oxidation reduction it does not hydrolyze when it enters water and.
When added to water chlorine dioxide forms chlorite ion which is also a very reactive chemical.
It is two and a half times heavier than air.
The chlorine dioxide molecule is small and volatile and is different from elementary chlorine.
Chlorine dioxide is a true gas at room temperature not a liquid meaning it will follow.
Chlorine dioxide is a gas at room temperature and can be dissolved within a solution.
Because of this surfaces treated with twinoxide don t need to be wiped off since there will be no residue is left after evaporation.
Coyote surprises wisconsin rapids residents as.
According to the u s.
Chlorine dioxide is used as a bleaching agent at paper manufacturing plants and in public water treatment facilities to make water safe to drink.
Summary chlorine vs chlorine dioxide.
2 clo 2 cloclo 3.
Chlorine chlorine physical and chemical properties.
Chlorine dioxide and ultraviolet light uv c can fit into your sanitation program to help alleviate these stresses and fully eliminate pathogens from within a production facility.
Chlorine dioxide casrn 10049 04 4.
It is a reddish to yellowish green gas at room temperature that dissolves in water.
Chlorine dioxide is a yellow to reddish yellow manufactured gas.
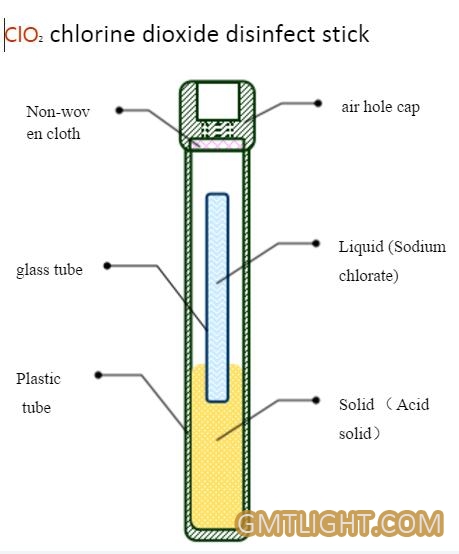
Room decontamination with chlorine dioxide gas.
Food and drug administration chlorine dioxide is a powerful bleaching agent which is a gas at room temperature.