Chlorine was first produced by carl wilhelm scheele a swedish chemist when he combined the mineral pyrolusite mno 2 with hydrochloric acid hcl in 1774 although scheele thought the gas produced in his experiment contained oxygen sir humphry davy proved in 1810 that it was actually a distinct.
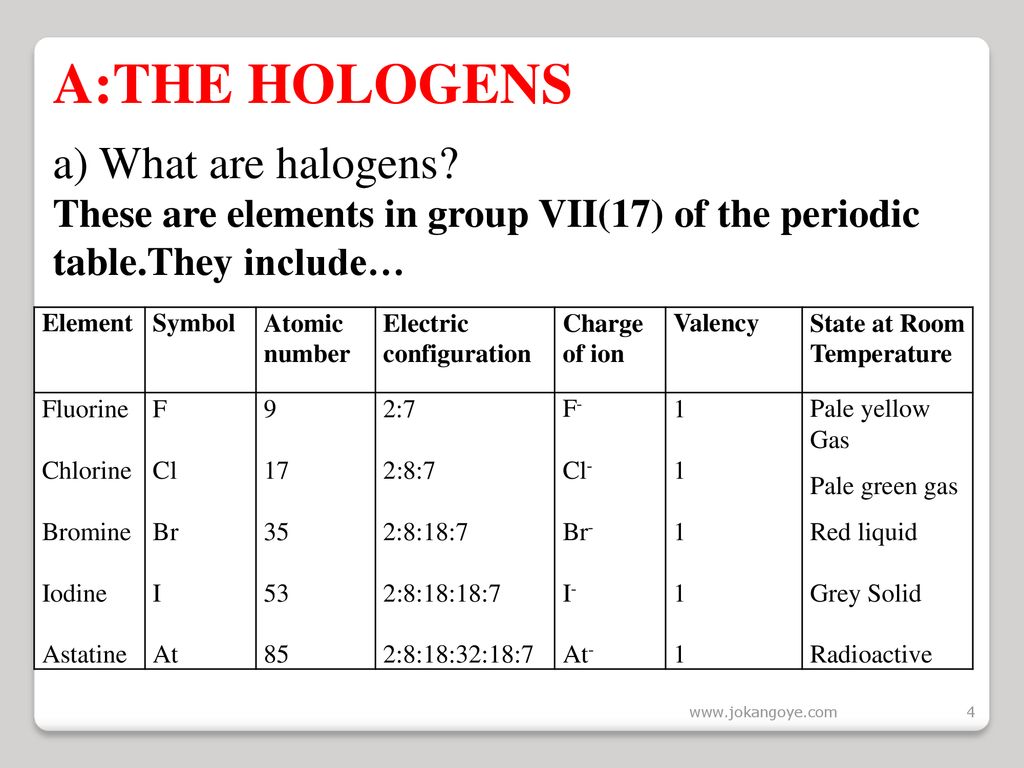
Chlorine state at room temperature.
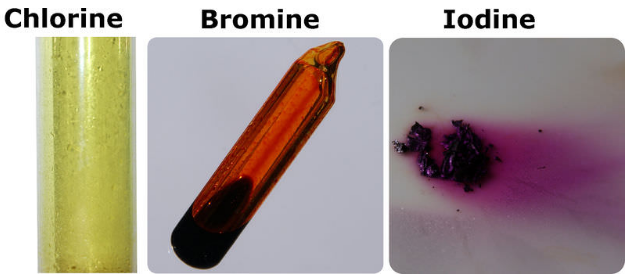
Chlorine has a yellowish green color at room temperature and is fatal if inhaled in large doses.
It is in the gaseous state.
Among the elements it has the highest electron affinity and the third highest electronegativity behind only oxygen and fluorine.
Chlorine is a yellow green gas at room temperature.
It is two and a half times heavier than air.
It becomes a liquid at 34 c 29 f.
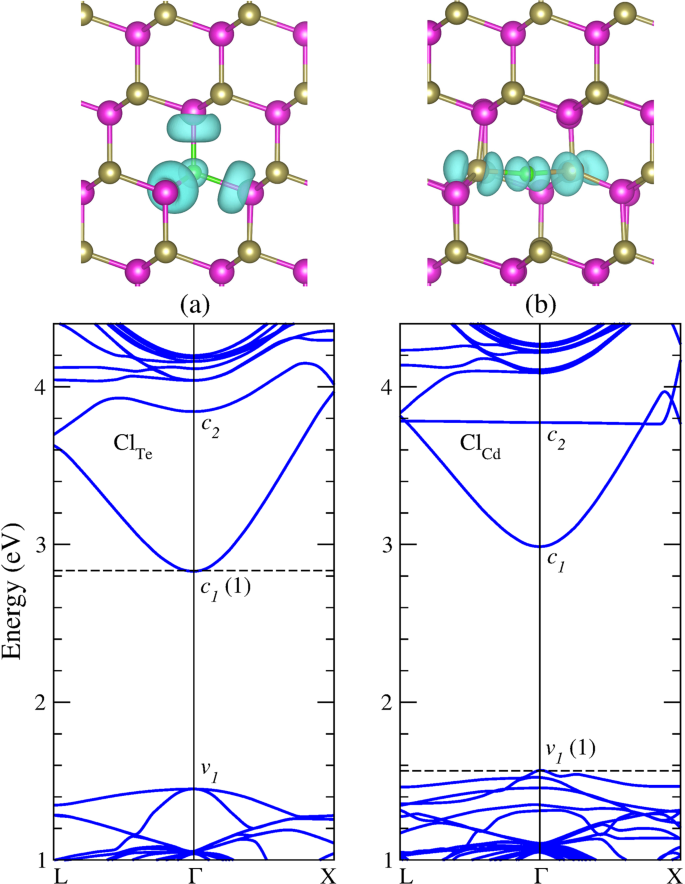
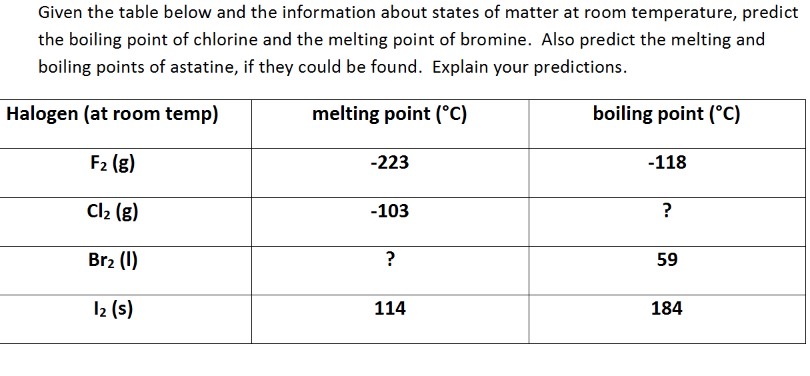
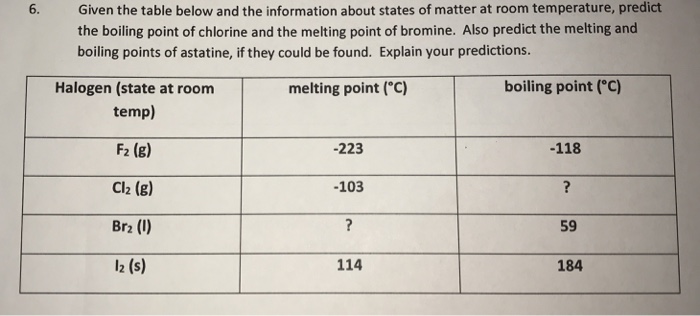
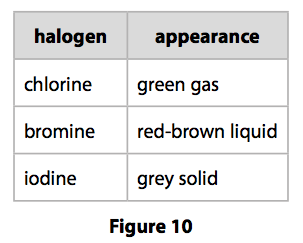
The table shows the colour and physical states of chlorine bromine and iodine at room temperature and pressure.
It is an extremely reactive element and a strong oxidising agent.
The boiling point of chlorine is 34 4 degrees celsius therefore at room temperature of about 20 23 5 degrees celsius it is a gas.
The density of chlorine gas is approximately 2 5 times greater than air which will cause it to initially remain near the ground in areas with little air movement.
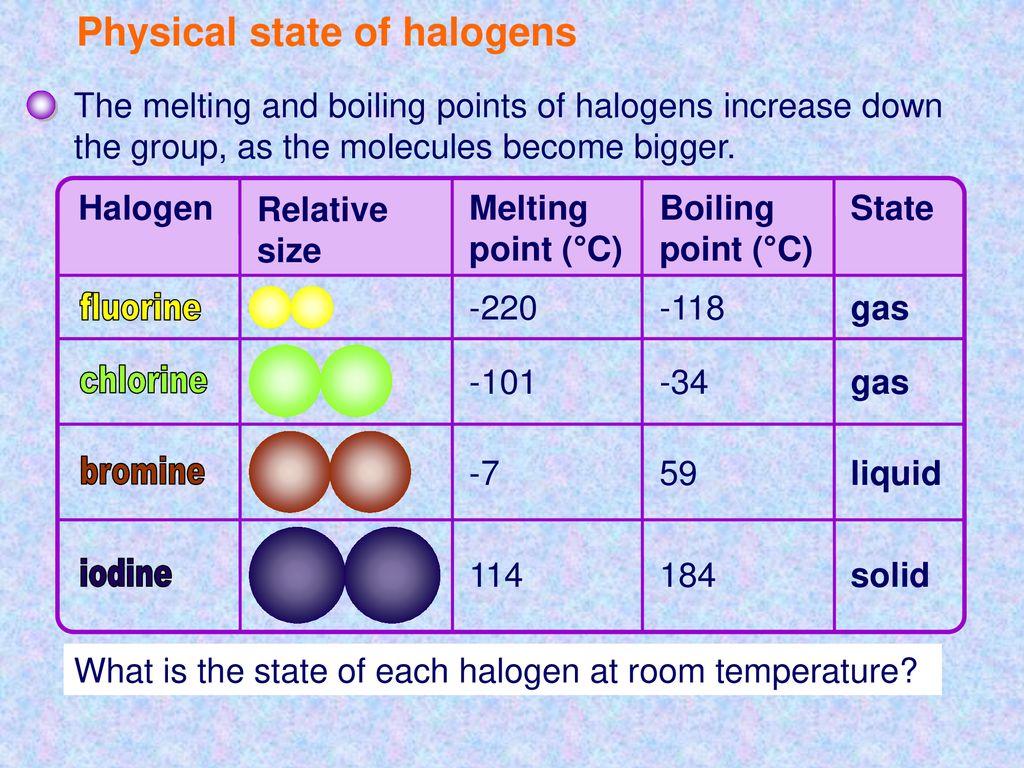
The melting points and boiling points of the halogens increase going down group 7.
It is an extremely reactive element and a strong oxidising agent.
Physical and chemical properties chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
Chlorine is a yellow green gas at room temperature.
Chlorine is a yellow green gas at room temperature.
Among the elements it has the highest electron affinity and the third highest electronegativity on the pauling scale behind only oxygen and fluorine.
Since it combines directly with nearly every element chlorine is never found free in nature.
ānswēr chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
It is a gas at room temperature don t listen to.